A spotlight on the treatment landscape of Myasthenia Gravis, an autoimmune disease
Putnam has a proven track record of delivering strategic support to pharma leaders in developing strategies for autoimmune diseases. In recent years, we have observed increasing clinical development activity in autoimmune diseases based on the increased understanding of disease pathogenesis and advent of multifactorial approaches including cell therapeutics (e.g., Tregs, Cytotoxic T cells, mesenchymal stem cells, etc.). The treatment landscape for Myasthenia Gravis is evolving with the recent approval of two neonatal Fc receptor blockers (VYVGART, RYSTIGGO) and two complement inhibitors (ULTOMIRIS, ZILBRYSQ). The treatment paradigm is expected to evolve further as multiple other neonatal Fc receptor blockers, complement inhibitors, and cell therapeutics are in clinical development. In this article, we provide an overview of the clinical development landscape for Myasthenia Gravis, highlighting the key modalities.
Myasthenia Gravis (MG)
Myasthenia Gravis (MG) is a rare disease that manifests as an autoimmune condition marked by skeletal muscles weakness and can either be localized (eye muscles) or generalized. Approximately 10-15% patients experience symptoms confined to the ocular muscles. In gMG, IgG autoantibodies are produced against proteins (AChR, MuSK and LRP4) involved in neuromuscular transmission. AChR gMG is most common (~80-85% cases) followed by MuSK (~5-8% cases) and LRP4 (~2% cases). Individuals testing positive for anti-AChR antibodies typically exhibit a greater degree of responsiveness to conventional treatment methods, whereas those testing positive for anti-MuSK antibodies often present with a more severe and resistant form of MG.
Current treatment landscape
Inhibitors of acetylcholinesterase (AChE) are effective to some degree in nearly all subgroups of MG, however, nearly all MG patients will require immunosuppressive therapy to achieve maximum therapeutic benefit. Corticosteriods in combination with azathioprine (or other ISTs) are used for first line immunosuppression. Patients not responding to initial immunosupression therapies are managed with biologics. While there are multiple approved biologics for AChR+ patients, there is only one approved therapy (RYSTIGGO) for MuSK+ patients.
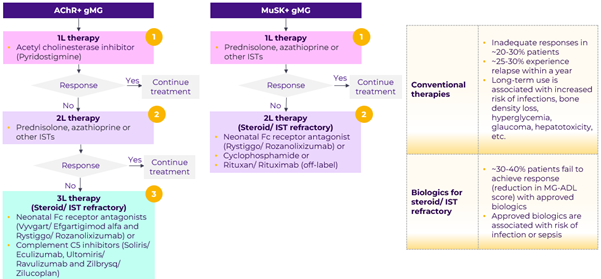
Patients may experience acute symptomatic flare-ups, known as myasthenic crisis, marked by severe symptoms such as respiratory or cardiac dysfunction, or severe infection. Such cases typically require admission to the intensive care unit and treatment with IVIg, plasmapheresis, or immunoadsorption.
Residual unmet need
Despite treatment, patients’ quality of life is significantly impacted – ~29% patients reported MG has a negative impact on their life. The current standard of care is associated with safety issues and ~30-40% patients fail to achieve adequate response. Moreover, there is a need for improved patient convenience (current SoC is either IV or SC). MuSK positive MG patients are more likely to remain steroid-dependent irrespective of additional treatment modalities and importantly complement inhibitors are not indicated in these patients.
The role of approved therapies in the treatment of myasthenia crisis (~20% of gMG cases) is unclear.
Emerging landscape
There are 16 assets in clinical development for gMG. Apart from therapies targeting FcRn and complement pathway, other novel MoAs in the pipeline include B-cell targeting therapies (CD19, CD20, BAFF/ BlyS inhibition) and CAR-T cell therapies. Innovation is focused on addressing unmet need for superior efficacy, safety, and patient convenience.

Neonatal Fc receptor blockers: Janssen/ Momenta’s nipocalimab has shown impressive efficacy in Phase II trial and offers convenience of Q2W dosing frequency. Batoclimab from Immunovant has demonstrated efficacy (MG-ADL responders – 86% vs. 56% placebo) in Phase II study, however efficacy is lower than the high benchmark set by VYVGART (MG-ADL responders – 68% vs. 30% placebo). While batoclimab’s SC formulation offers patient convenience, an increase in LDL cholesterol levels observed in thyroid eye disease trial and subsequent exclusion of high-risk CVD patients in Phase III study raises questions on safety.
Groundbreaking therapies: CAR-Ts targeting complete elimination of autoantibodies have the potential to significantly impact the treatment of gMG.
- Descartes-08/ Cartesian therapeutics, a first-in-class autologous mRNA CAR-T therapy is a promising pipeline asset. In a Phase IIa study, Descartes-08 demonstrated deep and durable clinical responses and was well tolerated in both AChR+ and MuSK+ gMG patients. Sustained clinically meaningful improvement was observed in 5 of 7 patients up to one year. Descartes-08 has several advantages over conventional DNA-based CAR-Ts – it is administered in an outpatient setting, does not need lymphodepletion and there is no risk of genomic integration.
- Cabaletta Bio is developing a MuSK specific chimeric autoantibody receptor-T (MuSK-CAAR-T) cell therapy for the treatment of MuSK-associated MG. The Phase I trial with Cabaletta’s MuSK-CAAR-T is underway.
Patient convenience: DNTH-103, complement C1s inhibitor (Phase IIb) from Dianthus Therapeutics is self-administered SC injection given every 2 weeks and BioCryst Pharma is developing an oral C5 inhibitor (Preclinical).
Improved safety: Current C5 inhibitors have boxed warning for serious bacterial infections. DNTH-103 targeting upstream complement C1s is expected to have a superior safety profile compared to downstream complement inhibitors. No bacterial infections were observed with DNTH-103 in a Phase I trial.
While promising, early data from pipeline assets still requires further clinical validation to fully assess their potential in gMG management. Future advancements in therapies targeting critical pathological mediators of gMG could enable a greater number of patients to reach minimal manifestation status and alleviate the disease burden.
Abbreviations
AChE: Acetylcholinesterase, AChR: Acetylcholine receptor, BAFF/ BlyS: B-cell activating factor/ B lymphocyte stimulator, CAAR-T: Chimeric autoantibody receptor- T cell therapy, CAR-T: chimeric antigen receptor-T cell therapy, CD: Cluster of differentiation, CVD: Cardiovascular disease, FcRn: Fragment crystallizable receptor, gMG: Generalized myasthenia gravis, IgG: Immunoglobulin G, ISTs: Immunosuppressants, IV: Intravenous, IVIg: Intravenous immunoglobulin, LDL: Low-density lipoprotein, LRP4: Low-density lipoprotein receptor-related protein 4, mAb: Monoclonal antibody, MAC: Membrane attack complex, MG: Myasthenia gravis, MG-ADL: Myasthenia Gravis Activities of Daily Living Scale, MoA: Mechanism of action, mRNA: Messenger ribonucleic acid, MuSK: Muscle-specific kinase, SC: Sub-cutaneous, siRNA: Small interfering ribonucleic acid, SoC: Standard of care
Sources:

Jump to a slide with the slide dots.
 Lori Klein, PharmD
Lori Klein, PharmD
How Medical Affairs is Embracing AI to Drive Precision and Impact
Explore how AI is reshaping Medical Affairs, driving precision, enhancing patient outcomes, and unlocking new opportunities for biopharma in 2025
Read more Rudiger Papsch
Rudiger Papsch
MAPS EMEA 2025: Driving Transformation and Excellence in Medical Affairs
Explore how MAPS EMEA 2025 redefined Medical Affairs - patient-centricity, AI, evidence generation & launch excellence take center stage
Read more Jo Ann Saitta
Jo Ann Saitta
Why AI Needs Humans: The Critical Thinking Advantage in Pharmaceutical Commercialization
AI speeds pharma insights, but human experts turn them into action. ClarityNav blends AI power with strategic, real-world expertise.
Read more