Putnam has a proven track record of delivering superior strategic support to pharma leaders in developing strategies for autoimmune diseases. In recent years, we have observed increasing clinical development activity in drugs targeting complement pathway based on the increased understanding of disease pathogenesis and multifactorial approaches. In this article, we provide an overview of the clinical development landscape for drugs targeting complement pathway highlighting the key targets and future trends.
Complement system and its regulation
The complement system is a vital part of the innate immune response, essential for combating infections and regulating inflammation. The complement system is a tightly regulated network of more than 30 proteins, largely designated by the letter C and number in the order of discovery (e.g., C1, C2, C3, etc.). These proteins are present as an inactive form in blood or as bound to membranes. Sequential cascade of enzymatic reactions cleaves and activates these proteins. Depending on the activation trigger, the complement cascade follows one of three pathways (Figure 1): the classical, lectin or alternative pathway leading to the formation of the membrane attack complex (MAC) and subsequent targeting of pathogens for destruction.
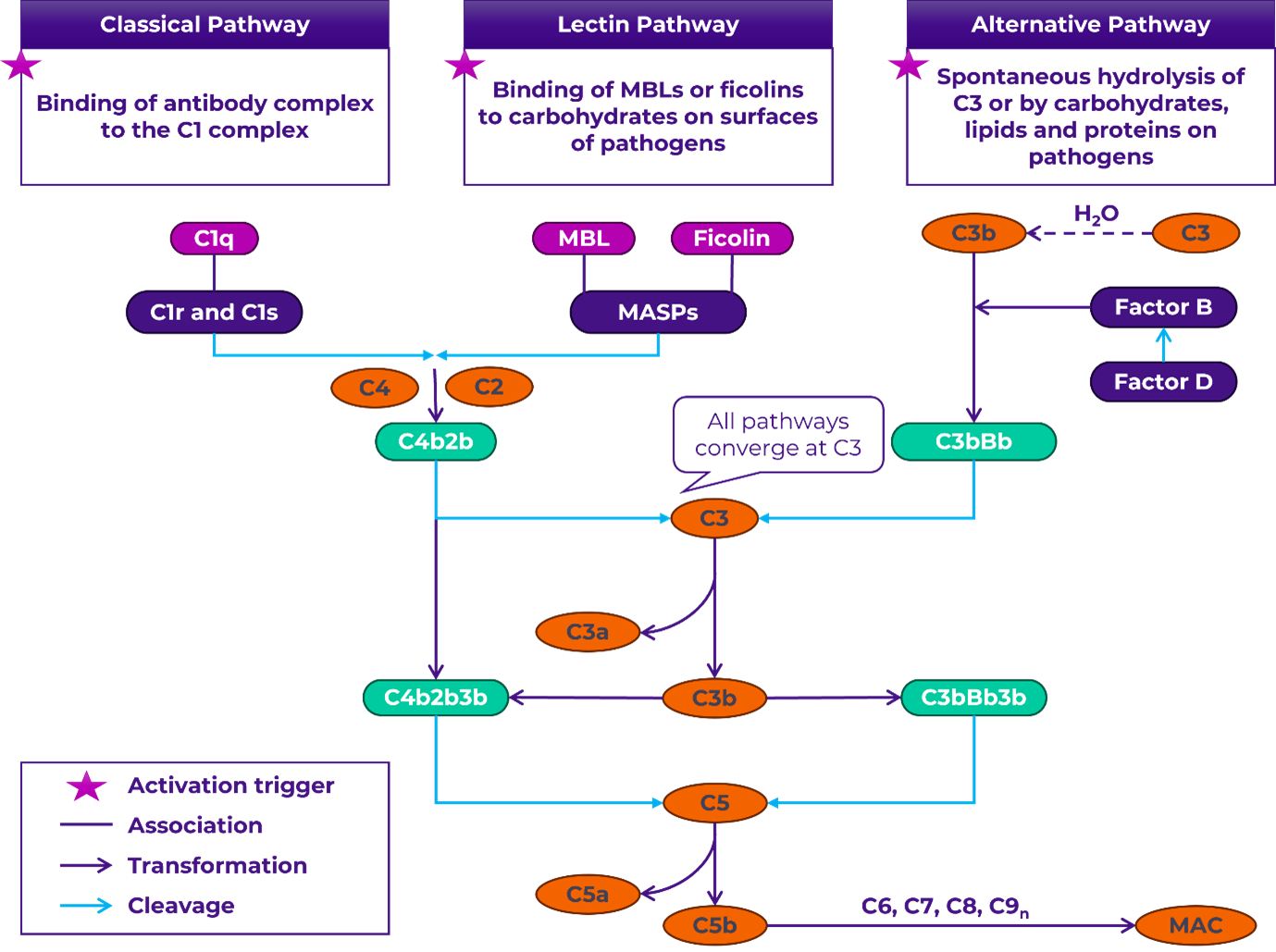
Figure 1. Complement system.
The complement system can be activated through the lectin, classical, or alternative pathways, depending on the trigger. The common pathway begins with the cleavage of C3 into C3a and C3b by C3 convertases. C3b binds to form the C5 convertase, which then cleaves C5 into C5a and C5b. C5b, along with C6, C7, C8, and C9, assembles into MAC, resulting in the direct lysis of target cells. Complement proteins are depicted in orange and convertases in green.
MBL: mannose-binding lectin; MASP: MBL serine proteases; MAC: membrane attack complex
Approved treatments for complement-mediated diseases
Complement-mediated diseases involve the dysregulation of the complement system, leading to various pathologies. Complement dysregulation may include both an inappropriate initiation of the cascade (atypical hemolytic uremic, paroxysmal nocturnal hemoglobinuria, geographic atrophy, etc.) or deficiencies (hereditary angioedema, CD55-deficient protein-losing enteropathy disease, etc.) in specific components or regulators. Currently there are 16 FDA approved treatments for various complement-mediated diseases (Table 1). The first drug approved for such conditions was Eculizumab, a humanized anti-C5 antibody, which received FDA approval in 2007 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH). The latest addition to this list is crovalimab, another anti-C5 antibody for the treatment of PNH.
Table 1. Current FDA approved complement drugs.
| Asset | Approved Indication(s) with year of approval |
| Common Pathway Target – C5 | |
| SOLIRIS (eculizumab) | PNH (2007), aHUS (2011), MG (2017), NMOSD (2019) |
| ULTOMIRIS (ravulizumab-cwvz) | PNH (2018), aHUS (2019), MG (2022), NMOSD (2024) |
| IZERVAY (avacincaptad pegol) | GA (2023) |
| VEOPOZ (pozelimab-bbfg) | CHAPLE disease (2023) |
| ZILBRYSQ (zilucoplan) | MG (2023) |
| PIASKY (crovalimab-akkz) | PNH (2024) |
| C1-esterase inhibitor (replacement therapy) | |
| CINRYZE (C1 esterase inhibitor [human]) | Prevention of HAE attacks (2008) |
| BERINERT (C1 esterase inhibitor [human]) | Treatment of HAE attacks (2009) |
| RUCONEST (C1 esterase inhibitor [recombinant]) | Treatment of HAE attacks (2014) |
| HAEGARDA (C1 esterase inhibitor subcutaneous [human]) | Prevention of HAE attacks (2017) |
| Common Pathway Target – C3 | |
| EMPAVELI, SYFOVRE (pegcetacoplan) | PNH (EMPAVELI-2021), GA (SYFOVRE-2023) |
| Common Pathway Target – C5aR | |
| TAVNEOS (avacopan) | AAV (GPA or MPA) (2021) |
| Classical Pathway Target – C1s | |
| ENJAYMO (sutimlimab-jome) | CAD (2022) |
| Common Pathway Target – C5a | |
| GOHIBIC (vilobelimab)* | Critical COVID-19 (2023) |
| Alternative Pathway Target – Factor B | |
| FABHALTA (iptacopan) | PNH (2023), IgAN (2024)# |
| Alternative Pathway Target – Factor D | |
| VOYDEYA (danicopan) | Extravascular hemolysis in PNH (2024) |
*FDA Emergency Use Authorization; #Accelerated Approval
AAV: Anti-neutrophil cytoplasmic antibody-associated vasculitis; aHUS: Atypical hemolytic uremic syndrome; CAD: Cold agglutinin disease; CHAPLE: CD55-deficient protein-losing enteropathy; COVID-19: Coronavirus disease; GA: Geographic atrophy; GPA: granulomatosis with polyangiitis; HAE: Hereditary angioedema; IgAN: Immunoglobulin A nephropathy; MPA: microscopic polyangiitis; MG: Myasthenia gravis; NMOSD: Neuromyelitis optica spectrum disorder; PNH: Paroxysmal nocturnal hemoglobinuria.
Emerging trends in complement therapeutics
Approved drugs are available for 10 indications, development is underway for at least 30 additional conditions. There are now 60+ new therapies in development including monoclonal and bispecific antibodies, peptides, proteins, RNA-based treatments, and gene therapies. Some emerging trends in complement therapeutics, reflecting a diverse and evolving landscape in the field, include:
Advancements in RNA-Based Approaches and Gene Therapy
RNA-Based Approaches offer innovative solutions to address the challenge of high target concentrations and drug doses in complement-mediated diseases by preventing the translation of complement proteins. RNA-based therapeutics offer high specificity with minimal off-target effects and do not activate Toll-like receptors, which helps to reduce the risk of unwanted immune responses. Currently, avacincaptad pegol, developed by Astellas for the treatment of geographic atrophy, is the only approved RNA-based therapeutic targeting the complement system. In addition to this marketed asset, there are five other RNA-based therapeutics in development (Table 2).
Table 2. RNA-based approaches targeting the complement system.
| Asset | Highest Phase of Development | Target | Company | Indication (s) | Modality | Comments |
| RG-6299 | Phase III | Factor B | Ionis/Roche | IgAN (Phase III), GA (Phase II) | ASO | ASOs, and RNAi therapeutics, act by silencing genes involved in complement activation |
| Cemdisiran + pozelimab | Phase III | C5 | Alnylam*/ Regeneron | PNH, MG | RNAi therapeutic | |
| AON-D21 | Phase II | C5a | Aptarion Biotech | Severe community-acquired pneumonia | L-RNA aptamer | RNA aptamers precisely bind to and inhibit C5a |
| ALXN-2030 | Phase I | C3 | AstraZeneca | Nephrology indication | siRNA | siRNAs, target and degrade mRNA of C3 to lower its levels |
| APL-3007 | Phase I | C3 | Apellis | Undisclosed | siRNA |
*Alnylam is responsible for development of cemdisiran monotherapy and has partnered with Regeneron for development in combination with pozelimab
ASO: Antisense oligonucleotide; GA: Geographic atrophy; IgAN: Immunoglobulin A nephropathy; MG: Myasthenia gravis.
Gene therapy has the potential to provide a cure with long-term benefits from a single treatment. However, challenges include ensuring safety, long-term efficacy and achieving effective delivery.
Currently, the only gene therapy in development is JNJ-1887 for the treatment of geographic atrophy. This one-time intravitreal gene therapy is designed to enhance the expression of CD59, a protein that inhibits membrane attack complex (MAC) formation thereby protecting retinal cells, and potentially slowing and preventing disease progression. Preliminary results from Phase I trials showed promising outcomes: 18.2% of subjects who received the lower dose of JNJ-1887 did not require re-treatment within the first 6 months. The therapy is now advancing to Phase II development.
Novel complement activation pathway inhibitors
Intervening at the initial stages of the complement pathway offers promising strategies for controlling uncontrolled activation of the classical, lectin, and alternative pathways (Table 3). By targeting an upstream regulator, it is possible to control the activation of all three pathways (classical, lectin, and alternative) simultaneously e.g., CSL-040. Blocking the common pathway by acting on a regulator or the convertase, will efficiently block all activation, amplification, and effector routes independent of the disease mechanism. This may pose a higher risk of blocking beneficial complement functions. Also, there are inhibitors in development that target specific pathways, aiming to halt pathological complement activation while retaining the system’s ability to defend against infections.
Table 3. Examples of novel complement activation pathway inhibitors in development.
| Asset | Highest Phase of Development | MoA | Company | Indication (s) | Comments |
| CSL-040 | Phase I | CR1 Fragment | CSL | Undisclosed | CR1 is a negative regulator of the complement system which binds to C3b and C4b to accelerate the decay of the C3 and C5 convertase complexes resulting in inhibition of all three complement pathways |
| Empasiprubart | Phase II | C2 inhibitor | Argenx | MMN, DGF, DM, CIDP | Inhibition of C2 prevents the formation of the C3 convertase and inhibits classical and lectin pathway activation |
| RLS-0071 | Phase II | C1Q/MBL inhibitor | ReAlta Life Sciences | HIE, aGVHD, Acute COPD exacerbations | By binding to C1q and MBL, it inhibits both classical and lectin pathways but does not inhibit alternative complement pathway which is important for immunity against pathogens |
| ADX-097 | Phase II | C3d-targeted factor H | Q32 Bio | Renal basket study (Phase II – LN, IgAN, C3G), AAV (Phase I) | ADX-097 is a bivalent fusion protein (C3d antibody–fH1-5) designed for targeted regulation of complement directly on the diseased tissues without long-term systemic blockade. It catalytically degrades alternative pathway convertases which leads to control of amplification loop and all 3 complement pathways |
| Classical Pathway | |||||
| ANX-005 | Phase III | C1Q inhibitor | Annexon Biosciences | GBS (Phase III), HD (Phase II), ALS (Phase II) | C1q inhibitors prevent the formation of C1 complex, which is essential for the subsequent activation of the classical pathway |
| ANX-007 | Phase II | C1Q inhibitor | Annexon Biosciences | GA | |
| Lectin pathway | |||||
| Narsoplimab | Phase III | MASP-2 inhibitor | Omeros | HSCT-TMA* (Phase III), COVID-19 and ARDS (Phase II), | MASP-2 is a key activator of the lectin pathway |
| SHR-2010 | Phase II | MASP-2 inhibitor | Jiangsu Hengrui | IgAN | |
| OMS1029 | Phase I | MASP-2 inhibitor | Omeros | Undisclosed | |
| Alternative pathway | |||||
| Zaltenibart (OMS906) | Phase II | MASP-3 inhibitor | Omeros | PNH, C3G , IC-MPGN | MASP-3 (a splice variant of MASP-1) activates factor D, a key regulator of the alternative pathway |
| GT-103 + pembrolizumab | Phase II (Investigator-sponsored trial) | Factor H inhibitor | Grid Therapeutics/ Merck# | Advanced non-small cell lung cancer | Factor H is a regulator of the alternative pathway; it binds to C3b and accelerates the decay of the alternative C3 convertase. By inhibiting factor H, GT-103 enhances complement-dependent lysis of tumor cells, modulates the adaptive immune response, and inhibits tumor growth |
*Ongoing discussions with FDA toward resubmission of BLA in HSTA-TMA; # Investigator-sponsored trial (NCT05617313), Merck and Grid are each providing their respective drugs and funding support
ALS: Amyotrophic lateral sclerosis; ARDS: Acute respiratory distress syndrome; aGVHD: Acute graft-versus-host disease; C3G: Complement 3 glomerulopathy; CIDP: Chronic inflammatory demyelinating polyneuropathy; COPD: Chronic obstructive pulmonary disease; COVID-19: Coronavirus disease; DGF: Delayed graft function; DM: Dermatomyositis; GA: Geographic atrophy; GBS: Guillain-Barré syndrome; HIE: Hypoxic-ischemia encephalopathy; HSTA-TMA: Hematopoietic stem cell transplant-related thrombotic microangiopathy; HD: Huntington’s disease; IgAN: Immunoglobulin A nephropathy; IC-MPGN: Idiopathic immune complex-mediated glomerulonephritis; LN: Lupus nephritis; MMN: Multifocal motor neuropathy; MoA: Mechanism of action; PNH: Paroxysmal nocturnal hemoglobinuria.
Conclusion
The involvement of complement activation in many conditions makes it an attractive target, however, the complexity of disease mechanisms and the varied contributions of complement to different pathologies pose considerable challenges in selecting a suitable target. Another important consideration is to select the optimal intervention points. Upstream interventions can prevent disease exacerbation early on but may compromise protective functions. In contrast, terminal interventions at the level of C5 activation, prevent cell damage and inflammation without directly disrupting danger sensing and opsonization, although they still risk affecting antimicrobial activity.
Approved treatments have significantly improved the management of complement-mediated diseases by targeting key complement mediators. As new therapies emerge, the landscape is becoming increasingly competitive. Therefore, it is crucial for drug developers to not only innovate but ensure that the clinical development plan supports regulatory approval, establishes meaningful and sustainable differentiation, and drives commercial adoption.
Reach out to Putnam to discuss your specific needs and questions. With our decades of experience across therapy areas and manufacturers, we can help you find custom solutions to these challenges as well as many others that may be faced during drug development and commercialization.
Sources
- West EE, et al. 2024 Jan 27;403(10424):392-405
- Zelek WM, et al. Mol Immunol. 2019 Oct;114:341-352
- Müller-Redetzky H, et al. 2020 Apr;132(4):795-807
- Nimjee SM, et al.Annu Rev Pharmacol Toxicol. 2017 Jan 6;57:61-79
- Ali Zaidi SS, et al. J Nanobiotechnology. 2023 Oct 18;21(1):381
- Rowe LW, Ciulla TA. Genes (Basel). 2024 Jun 1;15(6):720
- Hardy MP, Rowe T, Wymann S. J. Immunol. 2022;6:1-7
- Clarke JM, et al. J Clin Oncol. 2023 May;41(16):611-35: 9128
- Ballanti E, et al. Immunol Res. 2013 Jul;56(2-3):477-91

Jump to a slide with the slide dots.
 Varun Renjen
Varun Renjen
Four Key Themes Shaping Medical Affairs: MAPS Americas 2025
Explore key themes from MAPS Americas 2025—advancing health equity, AI, and collaboration in Medical Affairs.
Read more Rudiger Papsch
Rudiger Papsch
Conference Recap: MedAffairs Transformation 2025 (London)
Explore MedAffairs 2025 insights: AI, omnichannel strategy & impact driving pharma success and better patient outcomes.
Read moreAdvancing Health Equity in Life Sciences: Panel Recap
Industry leaders discuss breaking barriers in health equity, inclusive research, and patient access at Inizio’s NYC panel.
Read more
