Leveraging indirect treatment comparisons in joint clinical assessments: Navigating tight timelines with precision
In the evolving healthcare landscape, securing market access for new treatments often depends on how effectively their efficacy and safety compare with those of existing therapies. When direct head-to-head clinical trials are unavailable, as is frequently the case, indirect treatment comparisons (ITCs) provide a crucial methodology to fill the gap. These comparisons are essential for demonstrating the relative efficacy and safety of treatments when no direct evidence exists. In the context of joint clinical assessments (JCAs), for which timelines are tight and methodologic rigour is paramount, proper anticipation of the potential ITCs is indispensable.
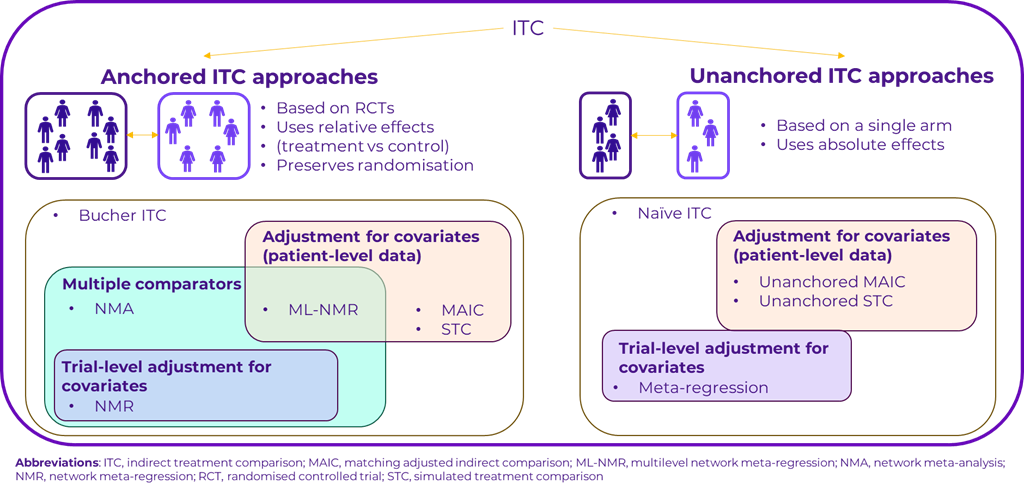
Anchored and unanchored ITCs
A core decision in any ITC is whether to use an anchored or unanchored approach. Anchored ITCs rely on randomised controlled trials and use a common control group to compare treatments. This method is preferred for its ability to preserve the integrity of randomisation, thereby minimising bias. Anchored approaches include methods like network meta-analysis, network meta-regression, matching adjusted indirect comparisons (MAIC), and multilevel network meta-regression (ML-NMR). MAIC and ML‑NMR adjust for patient-level covariates, providing a more refined and accurate estimate of the treatment effect.
Conversely, unanchored ITCs are typically used when randomised controlled trials are not available and are based on single-arm trials or observational data. These approaches do not have a shared comparator and rely on absolute treatment effects, making them more prone to bias, even when adjustments are applied. Unanchored approaches are not recommended by most health technology assessment (HTA) agencies and should only be used when anchored methods are unfeasible.
The role of ITCs in joint clinical assessments
Starting in 2025, joint clinical assessments (JCAs), which are governed by European HTA regulations, will require timely and methodologically sound evaluations of new treatments. ITCs are integral to this process, particularly when direct comparisons between treatments are absent. According to the Member State Coordination Group on Health Technology Assessment practical guidelines on direct and indirect comparisons (1), ITCs must adhere to strict guidelines to ensure that they produce valid and reliable estimates of treatment effect. The process involves in-depth feasibility assessment with careful selection of trials, examination of patient populations, and the development of an evidence synthesis plan accounting for differences across trials.
Furthermore, the Member State Coordination Group on Health Technology Assessment methodologic guidelines provides specific instructions on how to assess the data, adjust for confounders, and construct evidence networks for indirect comparisons (2). This guideline emphasises the need for methodologic consistency, particularly when handling multiple comparators in a single network. The inclusion of indirect evidence from larger networks is highlighted as potentially improving the certainty of evidence but also requiring careful scrutiny of assumptions to prevent bias.
Preparation is critical for JCA timelines
Given the tight timelines of JCAs, preparation is critical. Most of the workload for ITCs can be managed during the preparation phase, well before the final PICOS (Population, Intervention, Comparator, Outcome, Study Design) scoping is confirmed by the member states. A systematic literature review can identify the bulk of relevant studies, and preliminary data extraction sheets and programming codes can be created to allow for swift adjustments and updates once the specific PICOS are confirmed. Planning for a large scope is deemed less risky than updating an existing systematic literature review, and ITC is faster than starting from scratch with an analysis that might have been overlooked in the preparation process. This approach enables faster ITC implementation during the JCA, ensuring that results are delivered on time without compromising quality.
Conclusion
ITCs play a vital role in JCAs, allowing health technology developers to demonstrate the value of new treatments when direct comparison data are not available. Careful preparation using a wide scope for PICOS, following the methodologic and practical guidance provided by the Coordination Group on HTA, ensures that ITCs are conducted with the necessary methodologic rigor, paving the way for accurate, reliable comparisons that meet the stringent requirements of JCAs.
References:
- Member State Coordination Group on Health Technology Assessment. Practical Guideline for Quantitative Evidence Synthesis: Direct and Indirect Comparisons. Available from: https://health.ec.europa.eu/document/download/1f6b8a70-5ce0-404e-9066-120dc9a8df75_en?filename=hta_practical-guideline_direct-and-indirect-comparisons_en.pdf [Accessed 8 October 2024].
- Member State Coordination Group on Health Technology Assessment. Methodological Guideline for Quantitative Evidence Synthesis: Direct and Indirect Comparisons. Available from: https://health.ec.europa.eu/document/download/4ec8288e-6d15-49c5-a490-d8ad7748578f_en?filename=hta_methodological-guideline_direct-indirect-comparisons_en.pdf&prefLang=el#:~:text=synthesis%20(direct%20as%20well%20as%20indirect%20comparisons)%20are [Accessed 8 October 2024]
Jump to a slide with the slide dots.
 Clement Francois
Clement Francois
Refining the Parallels Between EMA Expansion and JCA Implementation: Quantitative Insights and Recommendations
Parallels between EMA & JCA reveal risks of imbalance in EU HTA. Learn how to ensure fair, inclusive JCA implementation.
Read moreAdvancing Health Equity in Life Sciences: Panel Recap
Industry leaders discuss breaking barriers in health equity, inclusive research, and patient access at Inizio’s NYC panel.
Read more Mike Harvey
Mike Harvey
How AI Is unlocking new insights from claims and EHR data in Medical Affairs
AI is transforming Medical Affairs, unlocking real-time insights from claims & EHR data to improve evidence generation, market shaping & patient care.
Read more
