Recent Trends in Rare Diseases
Research Contributors: Kellan Kim, Associate Consultant, Rohit Katoch, Research Consulting Senior Director, and Sushant Deswal, Research Consulting Manager
This is a preview of our latest whitepaper. Be sure to download the full piece below.
ABSTRACT:
The rare disease market has grown consistently over the past ten years and is expected to continue to grow, driven by regulatory incentives for orphan drugs and blockbuster commercial potential in high unmet- need diseases with little competition. Even with substantial pharma and biotech interest and investment, there remain hundreds of diseases with high unmet need for effective therapies. However, recent barriers have emerged, including increasing pricing pressure and emphasis on cost-effectiveness proven through comparative clinical trials. These have the potential to alter the historic attractiveness of drug development in rare diseases. This work details our observations on recent global trends within the rare disease space. We also draw implications for manufacturers and recommend strategies to maximize rare disease opportunities.
INTRODUCTION:
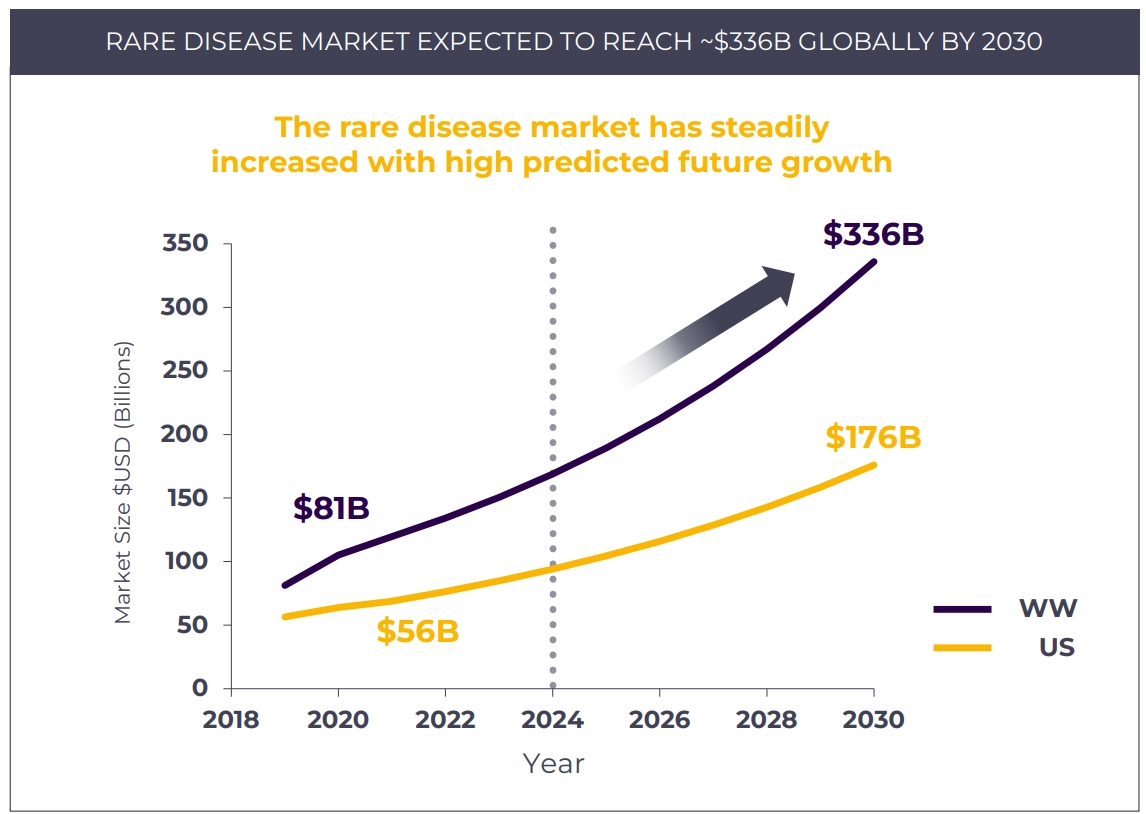
Overall Rare Disease Market Trajectory is Positive
Today, orphan drugs for rare diseases play a significant role in the global pharmaceutical market, accounting for 16% of pharma revenue. The global rare disease market has steadily increased in past years and is predicted to reach ~$336B globally by 2030. Notably, ex-US market growth is outpacing the US and is projected to comprise half of the global market by 2030.
Sources:
Rare Diseases Treatment Market Size, Share, Growth, Trends | Report 2022-2030 – BioSpace – Global.
Rare Diseases Treatment Market Size, Share Report, 2030 (grandviewresearch.com) – US. Rare disease numbers from National Organization for Rare Disease (NORD.org)
Note: The 500 pipeline products may be partially inclusive of 350 diseases with approved therapies
The top areas of rare disease investment are oncology, neurology, autoimmune, and ophthalmology. More broadly, the rise in biologics across the industry has also been a key driver of rare disease market growth in recent years, with notable modalities including immunotherapies, misfolded protein chaperone therapies, cell therapies, and gene replacement / gene-editing therapies. Therapies with technological innovation underpinning strong efficacy for high unmet need conditions and/or dosing convenience advantages have done particularly well, with 56 orphan drug designated therapies expected to attain >$1B in 2030 worldwide sales (34 outside of oncology) and 15 expected to achieve >$3B (7 outside of oncology).
Contact us.
Want to learn more? Fill out the form below to get in touch with our experts.
Jump to a slide with the slide dots.
 Mariah Hanley
Mariah Hanley
US vaccines trends and strategies for manufacturers
Discover insights into the evolving US vaccine landscape, trends, and strategic imperatives for successful novel vaccine launches. Download now!
Read more Vikram Gosain
Vikram Gosain
Key trends driving the emergence of targeted radiotherapeutics as an oncology therapeutic pillar
Targeted radiopharmaceuticals are transforming cancer treatment and may become the ultimate focal therapy.
Read more Karan Raje
Karan Raje
Simplify to amplify: “Declutter” your current Customer Engagement approach to maximize value
Learn how Putnam reviewed the paths took by five prominent biotech companies to establish itself as a successful commercial-stage organization.
Read more
