Revealing full value: Capturing equity impacts in economic evaluation using distributional cost-effectiveness analysis
Authored by: Mariia Dronova and Catriona Crossan
Equity has gained increasing interest from stakeholders in the healthcare industry, particularly since the initial outbreak of COVID-19, which exacerbated health inequalities worldwide. Health inequities arise because of a higher disease burden and poorer access to healthcare for individuals in more deprived groups, resulting in reduced life expectancy and quality of life, and a significant humanistic and economic burden for individuals, healthcare systems, and societies.
Healthcare decision-makers are concerned with several objectives that may compete with one another, such as maximising health while reducing health inequities or prioritising care for severe diseases. New medical interventions have the potential to either improve or harm health equity; however, this impact is not often accounted for in health economic evaluations. The absence of equity considerations in health economic evaluations presents a clear call for change so that equity can be weighted in the holistic decision-making process; beyond ethical considerations, investing in closing the inequity gap would substantially benefit individuals and economies.
Addressing health inequalities is a complex undertaking that may be achieved through coordinated public health policies across various areas, including the provision of healthcare overall and pharmaceuticals in particular. There have been growing policy concerns and calls for health technology assessment (HTA) agencies to formally incorporate equity assessments in their decision-making processes. The National Institute for Health and Care Excellence in England made tackling health inequalities 1 of 6 challenges in its strategy for the years 2021 to 2026, expressing interest in quantitative methods to incorporate equity assessment in economic evaluations. In the United States, the Institute for Clinical and Economic Review published a white paper to support the use of HTA methods incorporating equity assessment. In addition, a recent review revealed that equity is considered in 29 of 46 identified guidelines of HTA bodies worldwide.
Among the methodologic approaches allowing the assessment of equity impacts, distributional cost-effectiveness analysis (DCEA) is the most comprehensive quantitative framework to date. The objective of DCEA is to assess how health, before and after adoption of a new intervention, is distributed among population subgroups defined by equity-relevant attributes, also called social determinants of health (e.g., socioeconomic status, race, ethnicity, geography). This is achieved by assessing, at each step of delivery, how health outcomes and costs may differ between individuals based on the concept of the staircase of inequality.
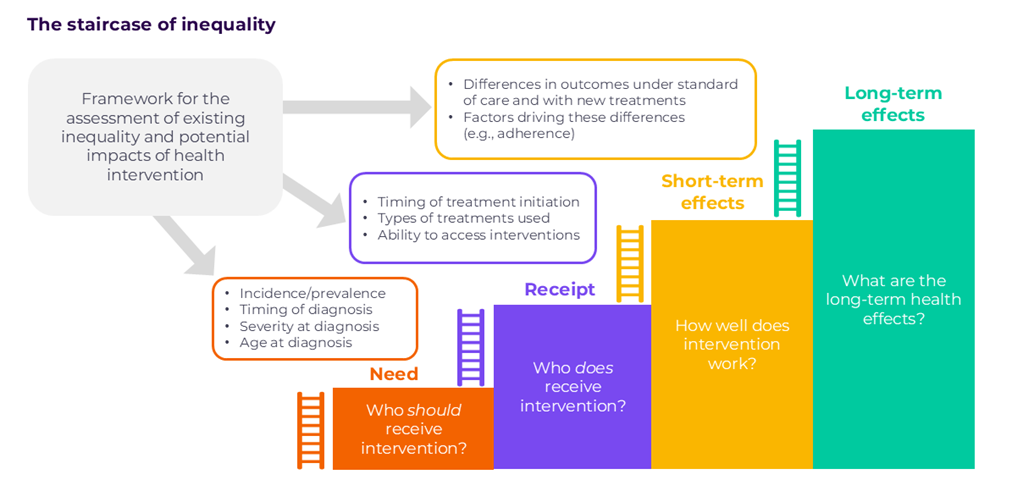
Although HTA bodies have expressed interest in quantitative approaches evaluating equity impacts, detailed guidance on the technical implementation and positioning regarding equity concerns are still in the development phase, given the novelty of these methods and additional data requirements. It has been recognised that DCEA is more data hungry than a standard cost-effectiveness analysis. At a minimum, epidemiology data stratified by equity-relevant attributes are required. Any other model inputs, driving differences in health outcomes and costs between equity-relevant subgroups, would contribute to building a more robust analysis. However, such evidence is limited for many countries because of a lack of data collection and reporting stratified by equity-relevant characteristics. These limitations can be overcome by using experts’ opinion where there are data gaps, and building on examples published in the literature for guidance on methods.
An intensive discussion on DCEA is ongoing, focusing on its methodologies, applications in healthcare decision-making, and potential to inform equity considerations in resource allocation. This is reflected in a growing number of peer-reviewed publications of DCEA studies on pharmaceuticals, which contributes to the dissemination of the method and paves the way for its implementation in HTA. Our Putnam experts are contributing to the development of the topic through a series of insightful publications and a recent open webinar on DCEA, aimed at fostering dialogue and collaboration among stakeholders in the healthcare community.
Further evolution of approaches capturing the value for equity in health economic evaluations can contribute to society’s goal of reducing health inequities, although some limitations must be addressed; the pharmaceutical industry can take a leading role in that. Partnering with governments and HTA bodies, industry can contribute to improving the evidence base required for DCEA through more inclusive clinical trials and stratified data collection in real-world evidence studies. Furthermore, building expertise in the area and a strong understanding of the requirements and challenges of DCEAs would equip the pharmaceutical industry to contribute to consultations with HTA bodies to shape future guidelines for incorporating equity in HTA, from data collection and reporting to methods of equity-augmented economic evaluations.
Interested in discussing equity in HTA and DCEA further? Come meet our experts at ISPOR Europe 2024 in Barcelona!
References
- National Institute for Health and Care Excellence. NICE Strategy 2021 to 2026. Available from: https://www.policyconnect.org.uk/sites/default/files/2021-05/aphg_nice_strategy_event_-_write_up_0.pdf [Accessed 10 October 2024]
- National Health Service. National Healthcare Inequalities Improvement Programme. Available from: https://www.england.nhs.uk/about/equality/equality-hub/national-healthcare-inequalities-improvement-programme/ [Accessed 10 October 2024]
- Agboola F, Whittington MD, Pearson SD. Advancing Health Technology Assessment Methods that Support Health Equity. Boston: Institute for Clinical and Economic Review; 2023
- Avsar TS, Yang X, Lorgelly P. Equity in national healthcare economic evaluation guidelines: essential or extraneous? Soc Sci Med. 2024;357:117220. https://doi.org/10.1016/j.socscimed.2024.117220
- Steijger D, Chatterjee C, Root W, Pavlova M. Challenges and limitations in distributional cost-effectiveness analysis: a systematic literature review. Int J Environ Res Public Health. 2022;20():505. https://doi.org/10.3390/ijerph20010505
Putnam publications
- Meunier A, Longworth L, Kowal S, Ramagopalan S, Love-Koh J, Griffin S. Distributional cost-effectiveness analysis of health technologies: data requirements and challenges. Value Health. 2023;26(1):60-63. doi: 10.1016/j.jval.2022.06.011
- Meunier A, Longworth L, Gomes M, Ramagopalan S, Garrison LP, Popat S. Distributional cost-effectiveness analysis of treatments for non-small cell lung cancer: an illustration of an aggregate analysis and its key drivers. PharmacoEconomics. 2023;41(8):1011-1025. https://doi.org/10.1007/s40273-023-01281-8
- Meunier A, Opeifa O, Longworth L, et al. An eye on equity: faricimab-driven health equity improvements in diabetic macular oedema using a distributional cost-effectiveness analysis from a UK societal perspective. Eye. 2024;38(10):1917-1925. https://doi.org/10.1038/s41433-024-03043-y
- Dronova M, Biundo E, Chicoye A, et al. The value of vaccination: capturing the impact of vaccination on health equity in health economic analysis. Value Health. 2022;25(12 suppl):S1. https://www.ispor.org/docs/default-source/euro2022/ispor-20eu-2022-20vov-20equity.pdf?sfvrsn=c39ef900_0
- Biundo E, Dronova M, Chicoye A, et al. Capturing the value of vaccination within health technology assessment and health economics–practical considerations for expanding valuation by including key concepts. Vaccines. 2024;12(7):773. https://doi.org/10.3390/vaccines12070773
- Meunier A, Dronova M. Distributional cost-effectiveness analysis (DCEA): capturing equity impacts in economic evaluation. Boston: Putnam; 2024. Available from: https://www.putassoc.com/insights/distributional-cost-effectiveness-analysis-dcea-capturing-equity-impacts-in-economic-evaluation/

Jump to a slide with the slide dots.
ESMO 2025 Insights: Emerging oncology trends highlighting the need for Precision Medical Affairs
At ESMO 2025, science set the pace, but Precision Medical Affairs defined the path, turning complexity into clarity and innovation into impact.
Read moreGetting Ahead of EU HTA: How our predictive PICO Simulator Capabilities Support Smarter Market Access
Predictive insight meets human intelligence, reshaping EU HTA readiness through smarter, data-driven evidence planning.
Read more Mariia Dronova
Mariia Dronova
Value of Equity in HTA: Current Guidelines and What to Expect
Equity in HTA is evolving. Explore current guidelines, future trends, and how Putnam advances equity methods in health assessments.
Read more
